As per the recent FDA security cautioning, breast implant recall might be connected to an expanded danger of malignant growth.
What Does the FDA Warning Say?
The FDA cautioning letter, distributed on Feb. 6, is coordinated towards medicinal services suppliers in different therapeutic fields, including corrective medical procedure, radiology, plastic medical procedure, obstetrics and gynecology, and oncology indicates that numerous ladies if you have bosom insets, you might later have a specific sort of embed related malignant growth.
Breast Implant Recall | Risk of Cancer Increased from Textured Breast Implants?
As indicated by the FDA letter, the specific kind of disease saw as associated with inserts is called breast implant associated-anaplastic large cell lymphoma (BIA-ALCL) and reports from patients as well as specialists demonstrate that the condition may at first happen inside the scar tissue encompassing your implant.
After bosom inserts are embedded into your chest, scar tissue starts to conform to the same and the scar tissue is known to regularly develop until it totally isolates the implant from the remainder of your bosom tissue; the same is inside this container where BIA-ALCL may create.
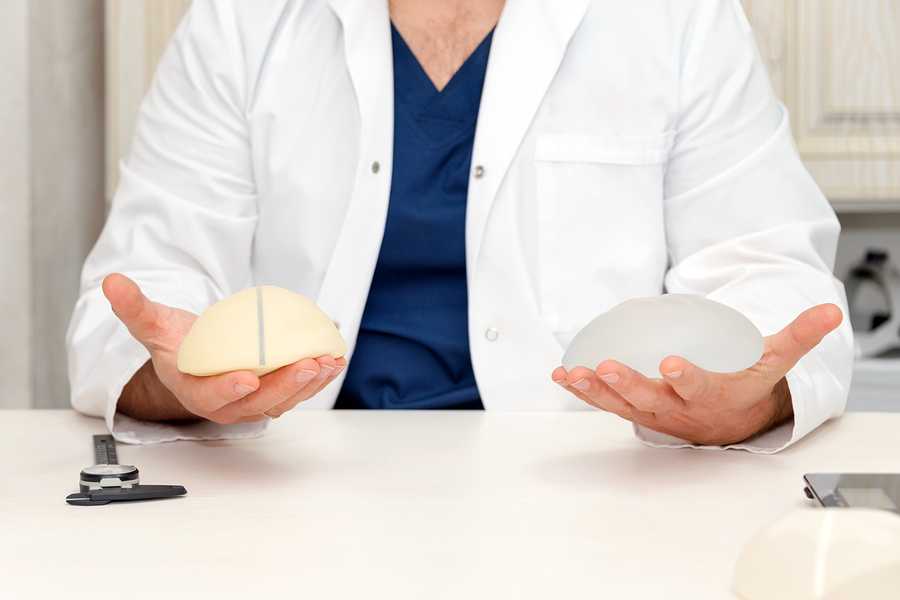
As per the FDA letter, patients with finished bosom inserts might be bound to create disease than patients with smooth inserts. Finished inserts have a harsh surface that elevates adherence to your encompassing tissue and this keeps your implants from moving around in the chest depression.
What is BIA-ALCL?
BIA-ALCL is a sort of malignant growth known as lymphoma and Lymphoma is known to start in your cells of the safe framework that battle diseases. BIA-ALCL isn’t a malignant growth of the bosom tissue and it by and large starts in your stringy scar tissue encompassing bosom inserts.
What are the Symptoms of BIA-ALCL?
Numerous patients who have been determined to have BIA-ALCL have had side effects including torment, expanding, irregular bumps in the bosom or armpit, bosom asymmetry, changes in bosom appearance, skin rash, liquid develop, or solidifying of the bosoms.
Specialists may check you for ALCL by utilizing an assortment of imaging tests, including ultrasounds and MRIs. Biopsies may likewise be important to affirm a malignant growth analysis.
Who May Be Affected?
BIA-ALCL is assessed to happen as regularly as one for every 3,817 patients; the greater part of the patients who have been determined to have BIA-ALCL have likewise had finished bosom inserts, despite the fact that there have additionally been reports of this malignant growth happening in patients who have smooth inserts. This means that, any sort of bosom implant may increase your exposure to the disease.
Also, finished bosom inserts are destined to be related with anapestic huge cell lymphoma. The FDA says that when bosom inserts are embedded into your body, they are set behind your bosom tissue or under your chest muscle, and a fibrous scar called a capsule conforms to your implant after some time and this scar tissue isolates your implant from the remainder of the bosom.
The FDA says that individuals if you are with finished bosom inserts, and then you are at a higher danger of creating ALCL in the scar capsule nearby your implant.
ABC clarifies that finished bosom inserts are mainstream in certain pieces of the world than others and in this way, if you are in one of those certain places then you might be at a higher danger of creating bosom embed related anapestic enormous cell lymphoma (BIA-ALCL) than others.
Further, there have been 572 cases all inclusive of BIA-ALCL and 33 deaths associated with BIA-ALCL around the globe.
Around the globe, wellbeing specialists have made a move to shield occupants from possible impacts of bosom inserts, especially for the finished ones. France has made a move to boycott few finished bosom inserts, and Canada has prohibited one brand, while Australia has started thinking about taking finished implants off the market, over similar concerns.
As far as it matters for its, the U.S. FDA has not restricted any bosom inserts and yet is examining the potential dangers related with inserts by observing reactions from wellbeing associations around the globe. Allergen bosom inserts are as of now at the core of this exchange and for the truth be told, in July, the FDA mentioned that Allergen, the producer of one kind of finished bosom embed, voluntarily reviewed and quit selling one of the embed models in the US in view of the danger of BIA-ALCL.
Notwithstanding malignant growth, if you have bosom inserts you may likewise experience reactions including joint inflammation, connective tissue malady, fibromyalgia, different sclerosis, sarcoidosis, and scleroderma.
Breast Implant Recall Lawyers
In the event that you have unpleasant or smooth bosom embeds and have been determined to have disease, including BIA-ALCL, you may fit the bill to take an interest in a bosom embed claim examination. You can contact Shamis& Gentile, P.A. if you would like to be a part of this class action investigation for breast implant recall of Allergans Textured Breast Implants.